Introduction to the Ethics Review Committee of Beijing GoBroad Hospital
The Ethics Review Committee of Beijing GoBroad Hospital (hereinafter referred to as the "Ethics Review Committee") was established on July 24, 2023, as part of the hospital's ethics review system. It collaborates with relevant management departments and researchers within the system, adhering to applicable laws, regulations, policy guidelines, and widely recognized ethical principles to safeguard the rights and safety of research participants.
Beijing GoBroad Hospital is responsible for the establishment and reappointment of the Ethics Review Committee, granting it the authority to conduct independent reviews and providing the necessary resources for its management and operations. The committee's mission is to ensure the scientific and ethical integrity of life sciences and medical research involving human participants, protecting their rights and safety while promoting research that meets high scientific and ethical standards. This, in turn, fosters public trust and support for such studies.
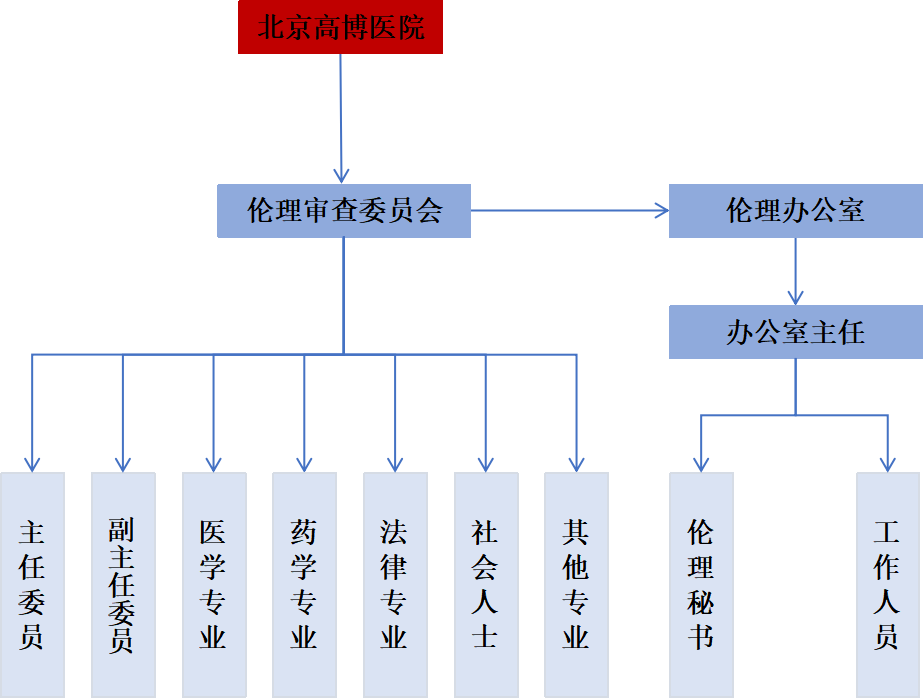
The Ethics Review Committee consists of one Chairperson, one Vice Chairperson, and 12 members. Additionally, it has an Ethics Office with a part-time Office Director and one full-time Secretary. The current members include experts from diverse fields such as medicine, pharmacy, non-medical disciplines, law, and social sciences. The committee maintains gender balance, with a 7:7 male-to-female ratio.
Organizational Structure
Facilitated Policy
The initial review project follows the pre-ethical review process. Pre-ethical review generally refers to the initiation of ethical review by the ethics review committee of the research center where the clinical trial is conducted, without obtaining the "Notice of Drug Clinical Trial from the Drug Evaluation Center of the National Medical Products Administration" or public announcement.
1. When a research project meets any of the following three conditions, the review opinion of the lead organization is recognized, and a simplified review process is adopted:Projects that participate in ethical mutual recognition, where the project review results of the main review unit in the alliance are recognized according to the relevant mutual recognition work mechanism.
2. Multicenter clinical studies with registration that have obtained approval from the lead organization.
3. Non-registration multicenter clinical studies where the lead unit is a tertiary hospital with outstanding scientific research capabilities in the relevant field.
Contact Us

Phone: 010-50847588 ext. 682

Email: bjgbll@gobroadhealthcare.com

Address: Ethics Office, 3rd Floor, Building 1, No. 4 Science Park Road, Changping District, Beijing

Postal Code: 102206















