Dr. Hu Kai | Unraveling the Complexity, Seizing Every Opportunity: A Salvage Treatment Case of Diffuse Large B-Cell Lymphoma After Failed Chemotherapy, Transplant, and CAR-T Therapy
Background
In recent years, the emergence of novel targeted therapies and CAR-T treatment has significantly improved the efficacy and prognosis of salvage therapy for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Many patients who once faced bleak prospects have even achieved remission or cure.
However, as a center specializing in relapsed/refractory lymphoma treatment, we have observed that with the wider adoption of these advanced therapies, some patients still experience relapse or disease progression after undergoing immunochemotherapy, autologous stem cell transplantation, and even CAR-T therapy, becoming what we term "super-refractory" cases.
Do these patients still have treatment options? Through extensive experience in managing relapsed/refractory lymphoma cases, we have found that these patients present highly complex clinical scenarios that cannot be addressed with a one-size-fits-all approach. Instead, meticulous analysis of their prior treatments and diagnostic history is required to identify potential opportunities and formulate highly individualized treatment strategies.
Here, we share the diagnosis and treatment journey of one such refractory patient.
Case Details
- Onset and Treatment at Other Hospitals
The patient, a 29-year-old male, experienced left lower back and sacral pain without obvious cause more than three years ago (May 2020). The pain was moderate in intensity, without radiating pain, numbness or weakness in both lower limbs, fever, night sweats, weight loss, chest tightness, or shortness of breath. After progressively worsening symptoms, he visited a local hospital, where a lumbar spine CT scan revealed multiple fractures and destruction of the left iliac wing, lumbar 5 transverse process, and lumbar 3/4 spinous processes, with surrounding soft tissue swelling. On September 3, 2020, a biopsy of the left iliac bone and lumbar spine was performed under local anesthesia.
The pathological biopsy result on September 10, 2020, showed diffuse medium-sized lymphoid cell infiltration, with the cells being round or oval, small nucleoli were faintly visible, mitotic figures were prominent, and a small amount of small lymphocytes and hematopoietic cells were present in the background.
Immunohistochemistry results: CD20 (diffuse +), PAX-5 (high +), TDT (-), CD3 (sporadic +), CD38 (+), CD138 (sporadic +), CD5 (sporadic +), CyclinD1 (-), CD23 (-), CD10 (-), Ki67 (45%).
FISH analysis: C-MYC, BCL-2, BCL-6 all negative. This suggested the possibility of bone-diffuse large B-cell lymphoma or large B-cell lymphoma involving the bone marrow.
Bone marrow aspiration and biopsy showed hyperplasia of hematopoietic tissue, with nucleated cells comprising 60% of the sample. Focal lymphoid cells formed nests. Immunohistochemistry showed: CD20 (focal +), CD3 (focal +), CD117 (-), CD61 (megakaryocyte lineage +). Special staining for reticulin fibers was positive (+/++). Based on immunohistochemistry results, B-cell lymphoma was suspected to involve the bone marrow.
Flow cytometry of bone marrow: Clonal B lymphocytes were present at 0.09% of nucleated cells, and were CD5- and CD10-.
Enhanced cranial MRI showed abnormal signals in the right frontal lobe and right corpus callosum knee, suggesting lymphoma infiltration. Left frontal white matter showed venous vascular malformation.
PET-CT (September 17, 2020): Multiple areas of bone absorption and destruction in the spine, right humerus upper segment, both clavicles, both scapulae, multiple ribs, sternum, pelvic bones, and upper segments of both femurs. Some areas showed soft tissue mass formation, with the left iliac bone being the most prominent. FDG uptake was abnormally high (SUVmax 26.7), and the lesion in the left iliac bone measured approximately 133mm x 92mm x 99mm.
Conclusion: Findings consistent with left iliac bone lymphoma involvement and multi-site bone involvement.
Diagnosis: Diffuse Large B-Cell Lymphoma (Stage IV-A, IPI score 3: Intermediate-high risk)
After confirming the diagnosis, the patient immediately started the standard first-line R-CHOP chemotherapy. After four cycles of chemotherapy, the evaluation showed partial remission. The patient then continued with two more cycles of R-CHOP chemotherapy and one cycle of Rituximab treatment. After completing the standard chemotherapy, disease progression was observed, entering the stage of refractory and relapsed lymphoma.
The patient was then referred to another hospital for further treatment. Upon admission, pathological consultation, FISH rechecking, and next-generation sequencing were performed. The results showed:
- Pathological consultation opinion: Non-Hodgkin’s B-cell lymphoma (non-germinal center phenotype), with a high likelihood of diffuse large B-cell lymphoma.
- Immunohistochemistry: Tumor cells CD20 (+), CD19 (+), CD22 (+), BCL-2 (+), MUM1 (about 40% +), CD21 (weak +, no FDC net shown), CD5 (-), CD10 (-), BCL-6 (-), C-MYC (-), CD30 (-), CD3 (-), Ki-67 LI (about 40%).
- Molecular pathology: EREB CISH (-).
- FISH: C-MYC, BCL2, BCL6 all negative.
- Next-generation sequencing: Mutations found in MYD88, CD79b, CD79a, EZH2, TMSB4X, CXCR4, DTX1, BCOR genes.
Based on these results, the patient began second-line treatment with the first cycle being R-DHAP chemotherapy combined with oral Ibrutinib. Due to the intensity of chemotherapy, the patient experienced severe bone marrow suppression, and Ibrutinib was not used consistently. After recovery of the blood counts, the tumor showed no remission, and disease stability was confirmed.
The patient then started the second chemotherapy cycle with high-dose R-MA (Methotrexate + Cytarabine) chemotherapy, followed by stem cell mobilization and collection. After this cycle of chemotherapy, the patient achieved partial remission. The hospital then recommended autologous stem cell transplantation combined with multi-target CAR-T therapy.
In June 2021, the patient received autologous stem cell transplantation with TBC (Thiotepa + Busulfan + Cyclophosphamide) as conditioning therapy. After stem cell reinfusion, autologous humanized CD19 CAR-T and CD22 CAR-T cells were sequentially infused. One month after the transplantation and CAR-T therapy, the patient’s blood counts recovered. However, one month after transplantation, disease progression was still noted: new abnormal signals were found in the right brain corpus callosum and right sacrum and ischium.
Subsequently, FC conditioning was given again, followed by the infusion of CD19 CAR-T cells (3.3 x 10^6/kg) and CD22 CAR-T cells (6.8 x 10^6/kg). One month after the infusion, the patient showed partial remission. To further consolidate treatment, the patient received another infusion of CD19 CAR-T and CD22 CAR-T cells.
In March 2022, three months after the last CAR-T cell infusion, the patient relapsed again. A routine MRI scan showed: new lesions with abnormal signals and enhancement in the right basal ganglia, thalamus, insula, hippocampus, and corona radiata. New lesions were also found in the gluteus maximus muscle. As the relapse also involved the central nervous system, high-dose methotrexate combined with Lenalidomide and Ibrutinib was administered at another hospital.
Concerned about the limited efficacy of chemotherapy, the patient sought further treatment and was referred to our department.
4o mini
ChatGPT:
Certainly! Here's the revised translation without bullet points:
Case Details
- Onset and Treatment at Other Hospitals
The patient, a 29-year-old male, experienced left lower back and sacral pain without obvious cause more than three years ago (May 2020). The pain was moderate in intensity, without radiating pain, numbness or weakness in both lower limbs, fever, night sweats, weight loss, chest tightness, or shortness of breath. After progressively worsening symptoms, he visited a local hospital, where a lumbar spine CT scan revealed multiple fractures and destruction of the left iliac wing, lumbar 5 transverse process, and lumbar 3/4 spinous processes, with surrounding soft tissue swelling. On September 3, 2020, a biopsy of the left iliac bone and lumbar spine was performed under local anesthesia.
The pathological biopsy result on September 10, 2020, showed diffuse medium-sized lymphoid cell infiltration, with the cells being round or oval, small nucleoli were faintly visible, mitotic figures were prominent, and a small amount of small lymphocytes and hematopoietic cells were present in the background.
Immunohistochemistry results: CD20 (diffuse +), PAX-5 (high +), TDT (-), CD3 (sporadic +), CD38 (+), CD138 (sporadic +), CD5 (sporadic +), CyclinD1 (-), CD23 (-), CD10 (-), Ki67 (45%).
FISH analysis: C-MYC, BCL-2, BCL-6 all negative. This suggested the possibility of bone-diffuse large B-cell lymphoma or large B-cell lymphoma involving the bone marrow.
Bone marrow aspiration and biopsy showed hyperplasia of hematopoietic tissue, with nucleated cells comprising 60% of the sample. Focal lymphoid cells formed nests. Immunohistochemistry showed: CD20 (focal +), CD3 (focal +), CD117 (-), CD61 (megakaryocyte lineage +). Special staining for reticulin fibers was positive (+/++). Based on immunohistochemistry results, B-cell lymphoma was suspected to involve the bone marrow.
Flow cytometry of bone marrow: Clonal B lymphocytes were present at 0.09% of nucleated cells, and were CD5- and CD10-.
Enhanced cranial MRI showed abnormal signals in the right frontal lobe and right corpus callosum knee, suggesting lymphoma infiltration. Left frontal white matter showed venous vascular malformation.
PET-CT (September 17, 2020): Multiple areas of bone absorption and destruction in the spine, right humerus upper segment, both clavicles, both scapulae, multiple ribs, sternum, pelvic bones, and upper segments of both femurs. Some areas showed soft tissue mass formation, with the left iliac bone being the most prominent. FDG uptake was abnormally high (SUVmax 26.7), and the lesion in the left iliac bone measured approximately 133mm x 92mm x 99mm.
Conclusion: Findings consistent with left iliac bone lymphoma involvement and multi-site bone involvement.
Diagnosis: Diffuse Large B-Cell Lymphoma (Stage IV-A, IPI score 3: Intermediate-high risk)
After confirming the diagnosis, the patient immediately started the standard first-line R-CHOP chemotherapy. After four cycles of chemotherapy, the evaluation showed partial remission. The patient then continued with two more cycles of R-CHOP chemotherapy and one cycle of Rituximab treatment. After completing the standard chemotherapy, disease progression was observed, entering the stage of refractory and relapsed lymphoma.
The patient was then referred to another hospital for further treatment. Upon admission, pathological consultation, FISH rechecking, and next-generation sequencing were performed. The results showed:
Pathological consultation opinion: Non-Hodgkin’s B-cell lymphoma (non-germinal center phenotype), with a high likelihood of diffuse large B-cell lymphoma. Immunohistochemistry: Tumor cells CD20 (+), CD19 (+), CD22 (+), BCL-2 (+), MUM1 (about 40% +), CD21 (weak +, no FDC net shown), CD5 (-), CD10 (-), BCL-6 (-), C-MYC (-), CD30 (-), CD3 (-), Ki-67 LI (about 40%). Molecular pathology: EREB CISH (-). FISH: C-MYC, BCL2, BCL6 all negative. Next-generation sequencing: Mutations found in MYD88, CD79b, CD79a, EZH2, TMSB4X, CXCR4, DTX1, BCOR genes.
Based on these results, the patient began second-line treatment with the first cycle being R-DHAP chemotherapy combined with oral Ibrutinib. Due to the intensity of chemotherapy, the patient experienced severe bone marrow suppression, and Ibrutinib was not used consistently. After recovery of the blood counts, the tumor showed no remission, and disease stability was confirmed.
The patient then started the second chemotherapy cycle with high-dose R-MA (Methotrexate + Cytarabine) chemotherapy, followed by stem cell mobilization and collection. After this cycle of chemotherapy, the patient achieved partial remission. The hospital then recommended autologous stem cell transplantation combined with multi-target CAR-T therapy.
In June 2021, the patient received autologous stem cell transplantation with TBC (Thiotepa + Busulfan + Cyclophosphamide) as conditioning therapy. After stem cell reinfusion, autologous humanized CD19 CAR-T and CD22 CAR-T cells were sequentially infused. One month after the transplantation and CAR-T therapy, the patient’s blood counts recovered. However, one month after transplantation, disease progression was still noted: new abnormal signals were found in the right brain corpus callosum and right sacrum and ischium.
Subsequently, FC conditioning was given again, followed by the infusion of CD19 CAR-T cells (3.3 x 10^6/kg) and CD22 CAR-T cells (6.8 x 10^6/kg). One month after the infusion, the patient showed partial remission. To further consolidate treatment, the patient received another infusion of CD19 CAR-T and CD22 CAR-T cells.
In March 2022, three months after the last CAR-T cell infusion, the patient relapsed again. A routine MRI scan showed: new lesions with abnormal signals and enhancement in the right basal ganglia, thalamus, insula, hippocampus, and corona radiata. New lesions were also found in the gluteus maximus muscle. As the relapse also involved the central nervous system, high-dose methotrexate combined with Lenalidomide and Ibrutinib was administered at another hospital.
Concerned about the limited efficacy of chemotherapy, the patient sought further treatment and was referred to our department.
Admission Details
The patient presented with mild dizziness and blurred vision, without fever, and normal bowel and urinary function. No other significant discomfort was reported.
Physical Examination upon Admission
The patient was alert and walked into the room. Vital signs: Temperature: 36.5°C, Pulse: 78 beats/min, Respiration: 20 breaths/min, Blood Pressure: 110/70 mmHg. The posterior pharyngeal wall showed congestion and edema.Laboratory Tests
- Complete Blood Count: White blood cells: 3.89×10^9/L, Hemoglobin: 113 g/L, Platelets: 98×10^9/L, Neutrophils: 2.26×10^9/L, Lymphocytes: 0.78×10^9/L
- Biochemistry: Lactate dehydrogenase: 140.00 U/L
- Hepatitis B Surface Antigen: Positive
- Special Protein Panel: Immunoglobulin IgG: 4.48 g/L
PET-CT
Deauville score: 5 points
Liver involvement: A lesion measuring approximately 3.2×2.6×3.7 cm, with a maximum SUV value of 22.4
Brain: Slightly low-density areas in the deep right temporal lobe and basal ganglia, suggesting lymphoma involvement based on MRI images
Multiple bone lesions: Bone absorption and destruction changes in multiple sites including the bilateral scapulae, clavicles, ribs, sternum, pelvic bones, and vertebrae. Some areas showed increased bone density, with the left iliac bone being the most prominent
Left gluteus maximus muscle: A lesion measuring approximately 1.2×1.6×1.5 cm, with a maximum SUV of 12.4
Diagnosis
Relapsed and refractory diffuse large B-cell lymphoma. Restaging: Stage IV, with central nervous system involvement. Genetic subtype: MCD.
Thoughts and Analysis
Reviewing the past treatment of this patient, one cannot help but feel disheartened. The patient had high-risk factors from the onset, particularly with widespread extranodal involvement and central nervous system involvement, which posed a great challenge to treatment. Although the patient did not completely fail standard first-line chemotherapy, the depth of remission was limited, as evidenced by the inability to achieve complete remission and the short-term relapse.
Subsequent genetic testing revealed the patient had the MCD subtype of diffuse large B-cell lymphoma, which genetically explained the patient's multiple extranodal involvement and early central nervous system invasion. For such patients, the standard treatment strategy after chemotherapy-induced remission is autologous stem cell transplantation (ASCT) as a bridge, but unfortunately, the patient could not achieve complete remission even after high-dose chemotherapy before the transplant. Historically, patients who could not achieve remission before autologous transplantation had a very high relapse rate.
To improve the success rate of transplantation in such patients, recent years have seen the combination of autologous transplantation with CAR-T therapy showing some promising results. As a result, some hospitals with the necessary conditions have begun to adopt this strategy in clinical practice. However, in this case, after undergoing autologous transplantation combined with multiple rounds of multi-target CAR-T therapy, the patient only achieved a brief partial remission, followed by relapse once again. It seemed like the treatment had reached an impasse, and the patient and family even considered discontinuing treatment.
After reviewing the patient's case in detail, I believe there may still be a chance for treatment. The reasons are as follows:
- The patient is young. Despite relapse, the period of disease control after previous treatments allowed the patient some time for recovery, maintaining a generally stable physical condition and normal blood cell counts. Therefore, the patient remains eligible for further treatment.
- Although the patient had relapse and progression, the overall tumor burden was not high upon admission, there were no large masses, and the lactate dehydrogenase levels were not elevated. The progression was not rapid, which provided some time and opportunity for treatment.
- As an MCD subtype, most patients have some efficacy with BTK inhibitors. The patient had not used BTK inhibitors regularly for an extended period in previous treatments, so it cannot be concluded that the patient is resistant to BTK inhibitors. Therefore, it remains an option for future treatment.
- The anthracycline-based chemotherapy drugs used by the patient earlier did show some efficacy, and it has been about a year since they were last used. In addition, the patient has never used etoposide, a common second-line chemotherapy drug for lymphoma, which suggests a lower likelihood of resistance to it.
- The patient still has preserved autologous hematopoietic stem cells, which provides a possibility for future treatment.
- Although the patient relapsed after CAR-T therapy, CAR-T treatment played a significant role in disease control throughout the process.
Taking these factors into consideration, we first decided to try a dose-adjusted TEDDi-R regimen for re-chemotherapy (reasons: it covers both central and non-central disease sites; it includes anthracycline-based drugs and etoposide, so the likelihood of resistance is low; it also includes BTK inhibitors, which may be effective for the MCD subtype). If this chemotherapy achieves the expected results, we will then attempt autologous stem cell transplantation combined with new-target CAR-T therapy for consolidation. To avoid repeating previous treatment failures, we require the patient to achieve a full and complete remission before this transplantation.
- Treatment in Our Hospital
Starting from April 2, 2022, the patient was treated with the DA-TEDDi-R regimen combined with targeted therapy (ibrutinib + rituximab + dexamethasone + liposomal doxorubicin + etoposide + temozolomide + long-acting GCS + intrathecal Ara-C) for two cycles. After the treatment, the PET-CT assessment showed complete metabolic remission (the first complete remission since the onset of the disease), with a Deauville score of 2-3. Comparing this with the PET/CT imaging from our center on April 1, 2022, the liver lesion significantly decreased and the high metabolic activity disappeared; the left gluteus maximus lesion also disappeared. There were localized bone resorption and destructive changes in multiple bones (bilateral scapulae, clavicles, ribs, sternum, pelvic bones, and vertebrae), with some areas showing increased bone density, particularly in the left iliac bone. The bone marrow glucose metabolism was diffusely elevated, suggesting reactive changes post-treatment, and we recommended further evaluation with a bone marrow biopsy.
After achieving the first treatment goal, we proceeded with the second autologous transplantation combined with CAR-T cell therapy as a consolidation treatment. This time, we used the TEAM regimen for conditioning (tirofiban + etoposide + cytarabine + mafosfamide) and prepared and infused CD19/CD20 dual-target CAR-T cells. The treatment process was relatively smooth. After the CAR-T cell infusion, the patient started experiencing fever on day 5, with a peak temperature of 39°C on day 8. This was diagnosed as cytokine release syndrome (CRS), classified as grade I, with no signs of immune effector cell-associated neurotoxicity syndrome (ICANS). CAR-T cell expansion was detected, and the patient's granulocytes engrafted on day 14 (July 5, 2022) after autologous hematopoietic stem cell infusion, and platelets engrafted on day 12 (July 3, 2022) after the same infusion. After the transplant, the patient was given oral ibrutinib as maintenance therapy.
5. Follow-up
The patient was subsequently followed up regularly, with imaging evaluations completed six months and one year after the transplant. It is gratifying to report that the patient has maintained complete remission throughout this period. The PET images are shown below:
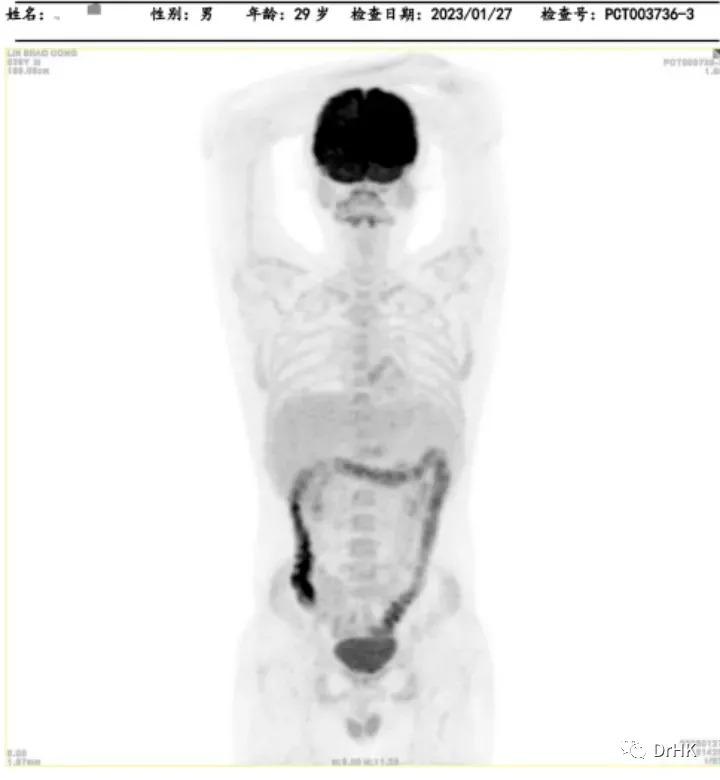
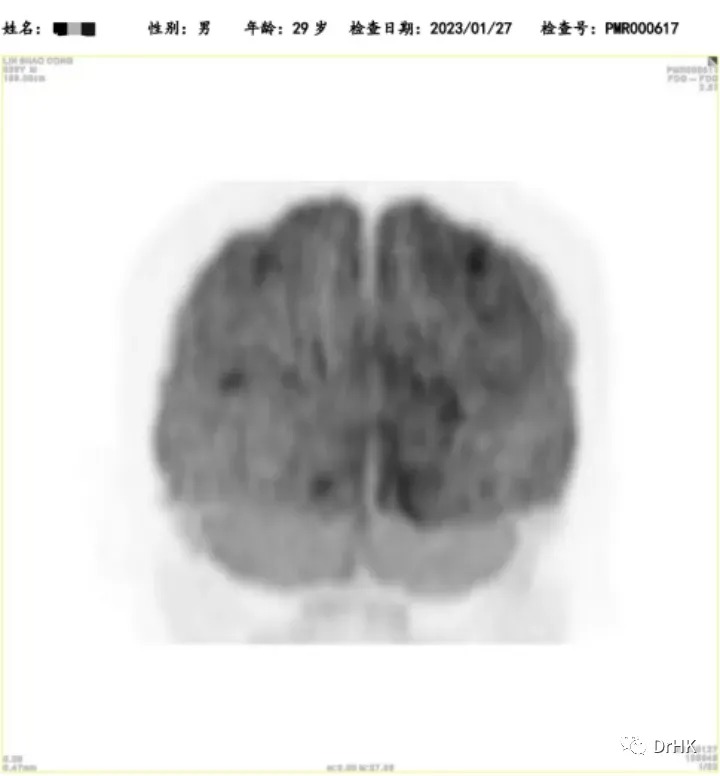
Six-Month Post-Transplant Imaging Evaluation
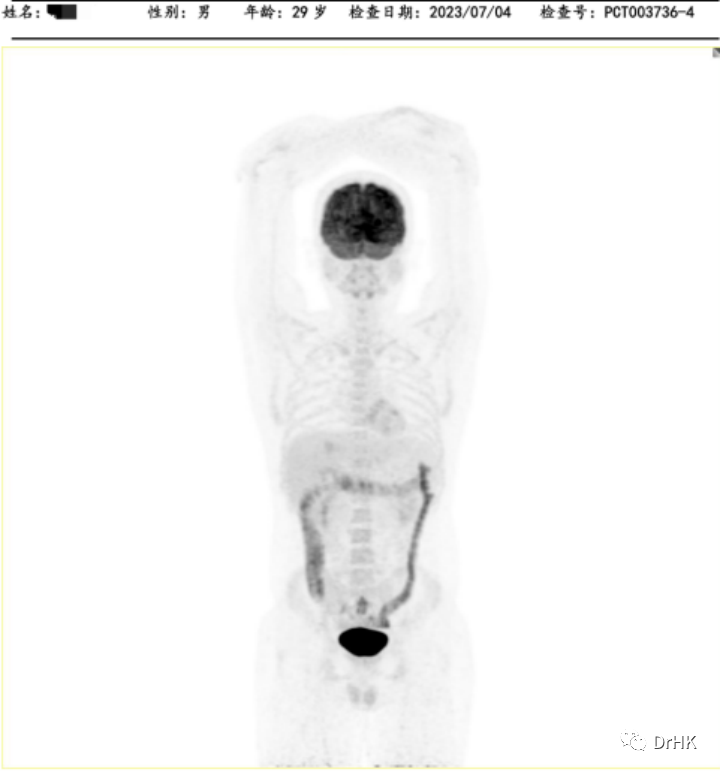
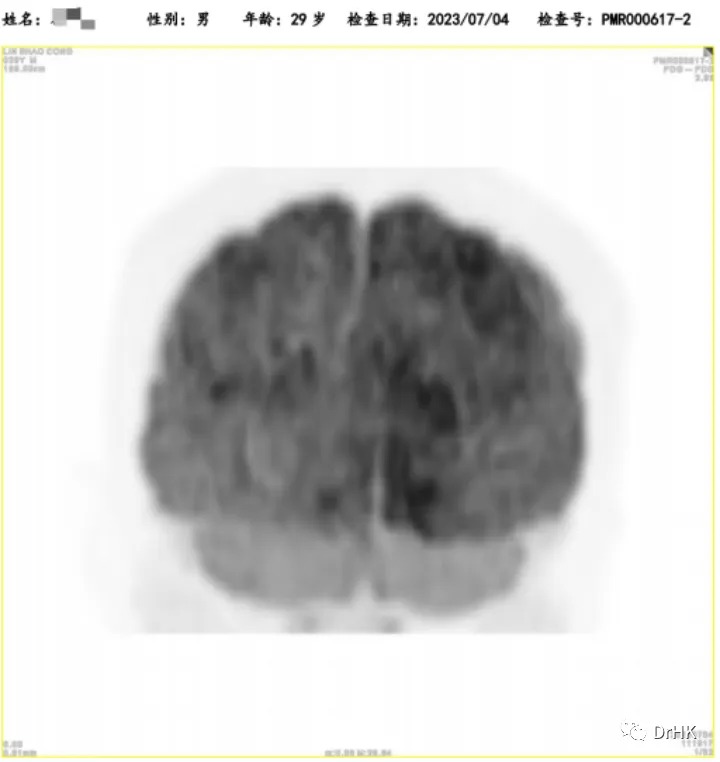
One-Year Post-Transplant Imaging Evaluation
Closing Thoughts
The advancement of treatment techniques has not only improved patient outcomes but also posed greater challenges for doctors, as we may face more difficult and complex cases of refractory and relapsed patients. This patient, after undergoing the most advanced treatments, still experienced a relapse, making it seem like the treatment had reached an insurmountable deadlock. Treating such patients is extremely difficult, and success is not always guaranteed; in fact, failure is more common. However, long-term professional experience has taught us that each case of refractory relapse carries its own unique tumor biology and clinical features. By carefully analyzing their characteristics and, especially, the successes and failures of previous treatments, we may find subtle clues that can guide future treatment and help us rediscover hope.
Expert Introduction
















