EHA 2024 | Professor Pan Jing: Latest Advances in CAR-T Therapy for T-Cell Lymphoid Tumors
CAR-T cell therapy is rapidly advancing in the field of hematologic malignancies, with significant progress made in the treatment of T-cell lymphoid tumors. However, challenges such as relapse, off-target effects, and viral infections remain to be addressed in order to further optimize the efficacy of CAR-T cell therapy. At the recently held 2024 European Hematology Association (EHA) Annual Meeting, the research conducted by Professor Pan Jing and her team from Beijing GoBroad Hospital garnered significant attention. Their two studies, which focus on the aforementioned challenges, were both selected for oral presentations. "Oncology Outlook – Hematology News" had the privilege of interviewing Professor Pan Jing on-site, where she shared and interpreted the findings of these two studies, with the aim of providing valuable guidance and practical suggestions for clinical CAR-T therapy in T-cell lymphoid tumors.
Study Overview
1、Background
Relapsed/refractory acute T lymphoblastic leukemia (r/r T-ALL) lacks effective salvage therapies and has a poor prognosis. Recently, CD7-targeted CAR-T cell therapy has shown efficacy in r/r T-ALL; however, CD7-negative relapse is relatively common. CD5 is expressed in over 80% of the blasts in T-ALL patients, and for those who relapse after CD7 CAR-T therapy or have low CD7 expression, CD5 can serve as an alternative target for CAR-T therapy.
2、Objective
This study aims to evaluate the application of donor-derived CD5 gene-edited CD5 CAR-T cells in the treatment of r/r T-ALL.
3、Methods
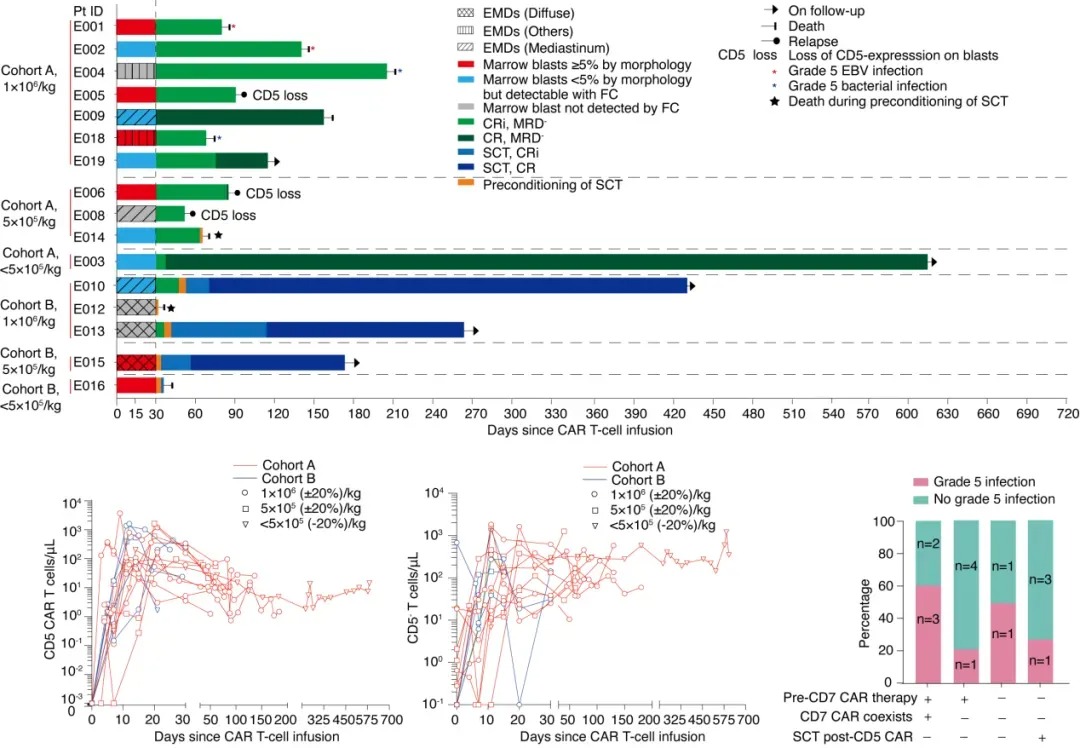
This single-center phase I study (NCT05032599) enrolled CD5+ r/r T-ALL patients who received CD5 CAR-T cells (using CRISPR-Cas9 to knock out the CD5 gene). The CAR-T cells were sourced from previous hematopoietic stem cell transplant (HSCT) donors (Cohort A) or newly matched donors (Cohort B) (Dai et al. Mol Ther 2021;29:2707-2722). A Bayesian Optimal Interval (BOIN12) design was used to explore the optimal biological dose (OBD) with two target doses of 1–2×10⁶/kg in both cohorts. If the number of CD5 CAR-T cells was insufficient, patients received a low dose of 0.5×10⁶ (±20%) /kg. The primary endpoint was safety, and the secondary endpoint was efficacy. For patients who achieved complete remission (CR) after CD5 CAR-T infusion, hematopoietic stem cell transplantation (SCT) was recommended as consolidation therapy. All patients or their guardians provided written informed consent prior to screening.
4、Results
From October 8, 2021, to March 21, 2023, 19 patients were enrolled, with 16 receiving CD5 CAR-T cell infusion, including 11 from Cohort A (7 receiving the target dose) and 5 from Cohort B (3 receiving the target dose). Of these, 10 patients (63%) relapsed after prior CD7 CAR-T therapy, with CD7 CAR-T cells detectable in 5 of them.
Adverse events (AEs) within 30 days included grade 3–4 cytopenia (100%, with 92% occurring before lymphodepletion), grade 1–2 cytokine release syndrome (75%), grade 1–2 neurotoxicity (25%), grade 1 skin graft-versus-host disease (GVHD) (69%), and one case of grade 3 infection (6%). No dose-limiting toxicities (DLTs) were observed.
Complete Remission with incomplete blood count recovery (CRi) at day 30 post-infusion was achieved in 100% of patients. As of July 31, 2023, the median follow-up time was 14.3 months (IQR 6.2–19.7). Four patients received SCT on day 35 (IQR 31–40) post-infusion, of whom three remained disease-free, and one died from fungal infection. Among the 12 patients who did not receive SCT (7 refused, 3 relapsed before transplant, 2 died during SCT pre-treatment), 2 maintained remission, 3 relapsed, 5 died from infections (2 from EBV and 3 from bacterial infections), and 2 died from thrombotic microangiopathy. Of all treated patients, half of the grade 5 infections occurred in those with coexisting CD7 CAR-T cells who did not receive SCT consolidation. Low-level CD7 CAR-T cells did not affect the expansion of CD5 CAR-T cells, and CD5 CAR-T cells dominated until the last follow-up. CD5+ lymphocytes decreased, while CD5- lymphocytes increased, with some originating from CD5 gene-edited products.
5、Conclusion
CD5 CAR-T cell therapy can effectively induce complete remission in r/r T-ALL patients. Despite significant efficacy, patients who did not receive subsequent SCT consolidation had limited duration of remission and faced an increased risk of long-term severe infections, especially those who had previously received CD7 CAR-T therapy. Combining CD5 CAR-T therapy with SCT consolidation may be a feasible approach to improving patient prognosis.
Efficacy, Expansion, and Safety of CAR-T Cells
Expert Interview
Oncology Insight - Hematology News
Interviewer:
At this year’s EHA conference, two of your studies were selected for oral presentations, achieving remarkable results. Could you please introduce the background and main outcomes of the study on CD5 CAR-T cell therapy for children and adults with relapsed/refractory acute T lymphoblastic leukemia (r/r T-ALL), using either prior transplant or new matched donors?
Professor Pan Jing:
The clinical trial for CD5 CAR-T therapy in relapsed/refractory T-ALL began in 2019. During our team's work on CD7 CAR-T, we found that some patients, particularly those treated with CD7 CAR-T, relapsed with CD7-negative cells after about six months. This off-target relapse is a common challenge in all CAR-T therapies. To address this, we partnered with Reindeer Biotech, as they were conducting preclinical studies on CD5 CAR-T and had published relevant research. This collaboration provided us with new insights, which we applied to our clinical research.Since 2019, this research has been ongoing, and the entire clinical trial has now concluded. At this EHA conference, we presented the comprehensive data. In this study, we enrolled 19 patients, 16 of whom successfully underwent CAR-T product infusion. It is worth noting that during the initial month of treatment, the risks faced by the patients were relatively low, with an overall response rate of 100%. Nearly all patients achieved remission with CAR-T therapy. The patients included in this study were primarily those who relapsed with CD7-negative cells after previous CD7 CAR-T therapy. Thus, CD5 CAR-T treatment is considered a salvage strategy for patients who failed CD7 CAR-T therapy. This background formed the basis for our research.
Interviewer:
Based on the results of this study, what is your outlook on the future of CD5 CAR-T cell therapy combined with hematopoietic stem cell transplantation (SCT) for consolidating treatment in r/r T-ALL? What are your next research plans in this area?
Professor Pan Jing:
In the past three years of research, we observed two key phenomena: First, regarding the off-target relapse issue in CAR-T therapy, especially in CD7 CAR-T treatment, CD5 CAR-T provides an effective supplement. Therefore, I believe that CD5 CAR-T currently serves as a complementary approach for patients who failed CD7 CAR-T therapy. However, in cases of T-ALL relapse, if a patient has higher expression of CD5 than CD7, CD5 CAR-T could be considered. From a safety standpoint, it is relatively safe as a bridge to transplant. We may explore further applications of CD5 CAR-T in the future, not only as a rescue measure but also as a means of selecting the most suitable CAR-T target based on the patient’s expression profile.Second, we noted differences in immune reconstitution between patients treated with CD5 CAR-T and those treated with CD7 CAR-T. In a healthy body, about 10% of T cells are CD7-negative. During T cell reconstitution, especially when no transplant is performed, CD7-negative T cells tend to expand rapidly. However, after CD5 CAR-T therapy, there are almost no normal CD5-negative cells in the patient’s body. In another study we presented at this conference regarding viral activation, we found that CD5 CAR-T-treated patients, who achieved remission, had very slow growth of CD5-negative cells. Most of these cells came from the knockdown results in the CAR-T product and were less functional compared to normal CD7-negative T cells. Based on this finding, we suggest that after CD5 CAR-T treatment, patients should undergo bridging transplantation for immune reconstitution. This is important because the lack of normal CD5-negative T cells could put the patient at risk for severe infections. This is another key message we hope to convey during this conference.
1. Background
Recently, chimeric antigen receptor (CAR) T-cell therapies targeting CD7 or CD5 have shown efficacy in patients with relapsed/refractory T-cell acute lymphoblastic leukemia/lymphoma (r/r T-ALL/LBL). However, severe infections, particularly cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections, remain significant risks during treatment. These infections may be related to the elimination of non-malignant T cells mediated by CAR-T cells.
2. Objective
This study aims to summarize the incidence of CMV and EBV infections in patients receiving CD7 or CD5 CAR-T cell therapy and to provide some risk management strategies.
3. Methods
This study is based on data from a phase I trial of autologous CD7 CAR-T therapy (NCT04840875), a phase I trial of donor-derived CD7 CAR-T therapy (ChiCTR2000034762), a phase II trial of donor-derived CD7 CAR-T therapy (NCT04689659), and a phase I trial of donor-derived CD5 CAR-T therapy (NCT05032599). All patients or their guardians provided written informed consent before screening. Infections were graded using the CTCAE 5.0 version. Univariate analysis used non-parametric tests, while multivariate analysis used logistic regression.
4. Results
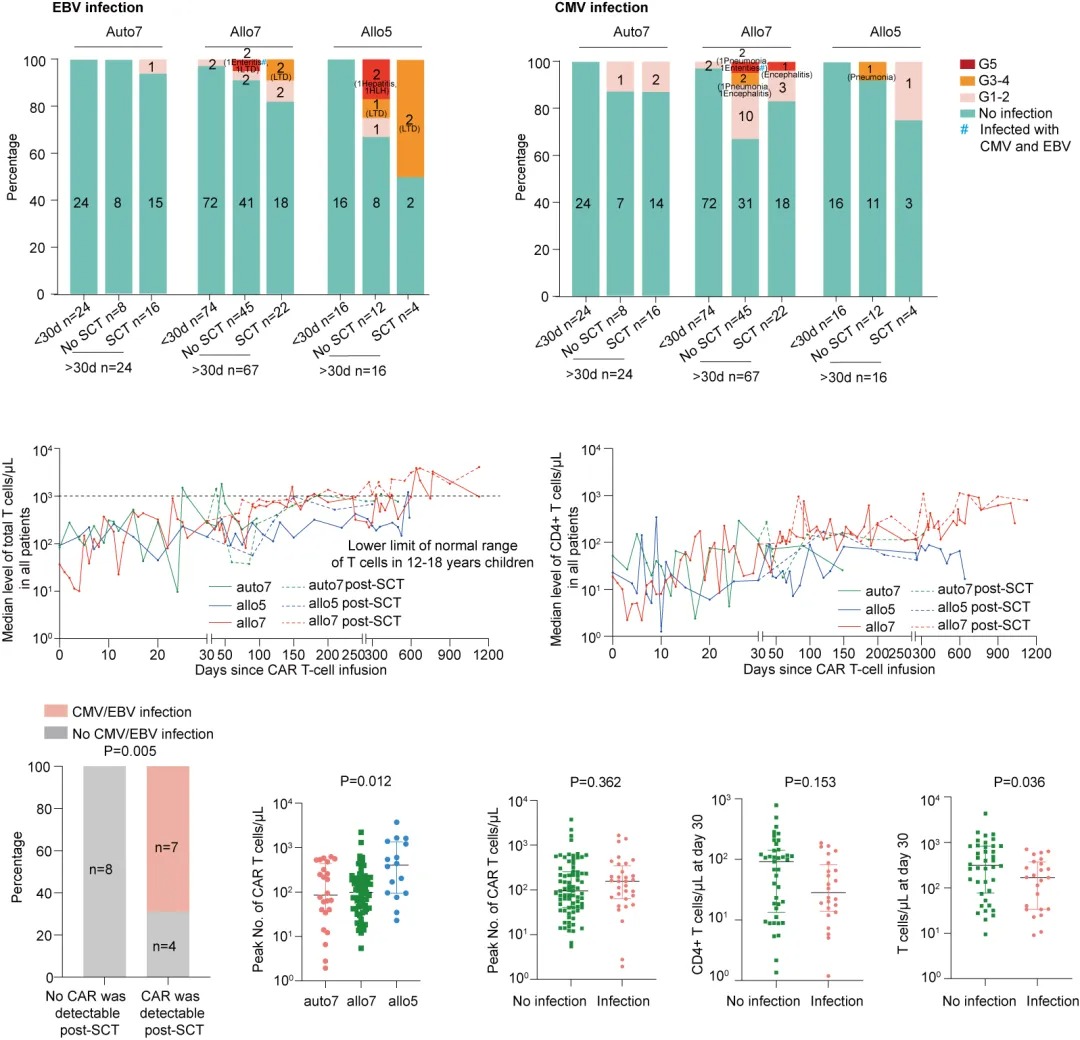
From July 2020 to September 2023, a total of 114 patients were included in the study. Among them, 25 patients (22%) experienced CMV infection, 17 patients (15%) had EBV infection, and 6 patients were co-infected with CMV and EBV.Within 30 days of CAR-T cell infusion, 2 patients (2%) had grade 1 CMV infections, and 2 patients (2%) had grade 1 EBV infections. After 30 days post-infusion, 107 patients continued follow-up (7 patients withdrew from the study). Among the 65 patients who did not receive consolidation stem cell transplantation (SCT), 14 patients (22%) had a recovery of total T-cell count to normal levels (in a median time of 1.3 months, IQR, 0.8–2.8), and 18 patients (28%) had a recovery of CD4+ lymphopenia to grade 2 or below (in a median time of 3.2 months, IQR, 2–4.3). Of these, 16 patients (25%) developed CMV infection (6 with severe infections) with a median onset of 2 months post-infusion (IQR, 1.3–3.3), and 8 patients (12%) developed EBV infection (4 with severe infections) with a median onset of 1.1 months post-infusion (IQR, 0.8–1.3).
Among the 42 patients who received SCT, 9 patients (21%) had a recovery of total T-cell count to normal levels (in a median time of 6.2 months post-transplant, IQR, 3.7–8), and 16 patients (38%) had a recovery of CD4+ lymphopenia to grade 2 or below (in a median time of 2.6 months post-transplant, IQR, 0.9–4.6). Of these, 7 patients (17%) developed CMV infection (1 with severe infection) with a median onset of 1.1 months post-transplant (IQR, 0.8–1.3), and 7 patients (17%) developed EBV infection (4 with severe infections) with a median onset of 2.1 months post-transplant (IQR, 1.2–2.7).
Almost all patients experienced T-cell and CD4+ T-cell depletion, which may contribute to the infections. The median total T-cell count and CD4+ T-cell count on day 30 post-infusion in infected patients were 135/μL (IQR, 31–259) and 15/μL (IQR, 9–22), respectively, which were lower than in non-infected patients, whose median total T-cell count was 302/μL (IQR, 36–616) (P=0.036) and CD4+ T-cell count was 79/μL (IQR, 20–145).
Furthermore, we observed that the peak CAR-T cell expansion in the CD5 CAR-T treatment group was higher than that in the CD7 CAR-T treatment group (P=0.012), although the peak CAR-T cell expansion was similar between infected and non-infected patients (P=0.362). Post-transplant, CAR-T cells were detectable in 11 patients, of whom 7 had viral infections, suggesting that post-transplant infections may also be related to the persistence of CAR-T cells.
Multivariate analysis of variables such as the peak CAR-T cell expansion, total T-cell count on day 30, and CD4+ T-cell count on day 30 revealed that total T-cell depletion on day 30 was independently associated with viral infections (P=0.029).
5. Conclusion
Most patients treated with CD5 and CD7 CAR-T cells experience persistent T-cell depletion. The study found that T-cell depletion is an independent risk factor for viral infections. Therefore, to promote T-cell recovery, consolidation transplantation after CAR-T cell therapy is recommended. Additionally, total T-cells typically recover within six months post-SCT, meaning that early post-SCT infection risks remain. Some infections occurring post-SCT may be related to the persistence of CAR-T cells. Implementing a reliable "suicide switch" in CAR-T products could improve the safety of CAR-T cell therapy for T-ALL in future studies.
EBV and CMV Infections and T-cell/CD4+ T-cell Levels After CAR-T Cell Infusion
Expert Interview
Oncology Outlook - Hematology News
Another study discussed the CMV/EBV infections in CAR-T treatment for relapsed/refractory acute T-cell leukemia/lymphoma (r/r T-ALL/LBL). Could you share how many patients in the study experienced CMV and EBV infections? At what stage after CAR-T infusion did these infections typically occur? What risk management strategies has your team proposed for these infections?
Professor Pan Jing: Actually, this study is part of the same series as our previously mentioned CD5 CAR-T and published CD7 CAR-T research. Currently, the major challenge in this field is verifying whether these drugs are effective for r/r T-ALL and whether they can be used as standalone therapies or must always be combined with bridge transplantation for curing patients. To answer these questions, we need to follow up with patients over a long period and assess T-cell function. In this CAR-T treatment for T-ALL study, we reviewed four studies: three involving CD7 CAR-T and one involving CD5 CAR-T, with a total of 114 patients enrolled. About half of these patients received bridge transplantation.
First, we focused on whether patients had any risks during the CAR-T treatment response phase (within 30 days). According to our data and data from other domestic centers, the safety within these 30 days is relatively high. Of the 114 patients, only three experienced infections, and their symptoms were mild, alleviating quickly with antiviral treatment. We further analyzed the data by dividing patients into bridge transplantation groups and non-bridge transplantation groups. Patients in the bridge transplantation group, including those undergoing a second transplantation, had an overall infection risk because immune recovery occurs within six months after transplantation. In contrast, non-bridge transplantation patients, particularly those with severe hematological toxicity before treatment, were more likely to experience serious infections after 30 days post-CAR-T treatment. This is because severe infections require immune dysfunction and time to develop.
Our long-term follow-up data showed that non-bridge transplantation patients had a very slow recovery of T-cell function, taking nearly a year to reach levels close to those seen after CD19 CAR-T treatment. Therefore, for patients who have very low blood counts and severe hematological toxicity after CD7 CAR-T or CD5 CAR-T treatment, we recommend early bridge transplantation to facilitate rapid T-cell recovery and avoid infections. For patients after CD5 CAR-T treatment, we particularly recommend immediate bridge transplantation because the CD5-negative T-cells in their bodies mainly come from the CAR-T product. For this, we may consider splitting the CD5 CAR-T product into two parts: one for CAR-T and one for CD5-negative T-cells, providing patients with enough CD5-negative T-cells to support immune reconstruction. This is one of the potential directions for our future research.
Oncology Outlook - Hematology News
In the study, you observed that infections occurring after SCT might be related to the persistent presence of CAR-T cells. What implications does this finding have for the future safety of CAR-T treatments? What is your view on the necessity of implementing "suicide switch" technology in CAR-T products?
Professor Pan Jing: This is not just a phenomenon observed in our center but also in multiple centers nationwide. T-cell CAR-T therapy has been in practice for nearly two to three years, especially in large transplant institutions where we frequently communicate. One issue we’ve observed is that if patients do not undergo bridge transplantation—for example, after CD7 CAR-T treatment and using their own stem cells for reinfusion—CAR-T cells almost do not diminish, but in such cases, patients may face high risks of viral activation and sepsis later on. Our team also presented this in detail at the EHA conference.
For patients undergoing bridge transplantation, following the conventional CD19 CAR-T treatment protocol, we expect the standard transplant and pre-treatment process to eliminate CAR-T cells. However, this is not always the case. Not all CAR-T cells disappear. For instance, in yesterday's Poster session, a team from the Western Theater General Hospital in China, led by Professor Su Yi, presented data showing that a small portion of patients still had CAR-T cells after transplantation. We’ve observed this phenomenon in our center and other transplant centers in Chengdu, Shanghai, and Wuhan. These patients later developed severe immune deficiencies. Therefore, we recommend that for patients after transplantation, especially those at less experienced transplant centers, it is crucial to monitor the patient’s T-cell phenotype post-transplant. This is because, after one month post-transplant, only 48% of patients' T-cells have recovered to a normal state with both CD7 and CD5 positivity, indicating that T-cell recovery is a slow process. If T-cells do not return to normal, the patient’s risk of infection will significantly increase.
Furthermore, regarding the choice of transplantation preconditioning, although we have not yet obtained complete data, we have observed that patients treated with TBI have a lower likelihood of CAR-T cell survival post-transplant. On the other hand, non-TBI patients tend to have higher CAR-T cell survival rates. This is a result we have privately discussed with other centers. As for making bridge transplantation safer, we believe it requires further support from large data studies. In clinical trials of T-cell CAR-T, we have tried using switches to control CAR-T cell activity, but unfortunately, almost all reported switch methods have been ineffective. Therefore, our next focus will be developing a more suitable and clinically effective switch to rapidly deactivate CAR-T function in the event of infection, restoring normal immune function and thus reducing the risk of infection. This is one of the basic and translational research directions we will pursue in the future.
Expert Introduction

(来源:《肿瘤瞭望–血液时讯》编辑部)















